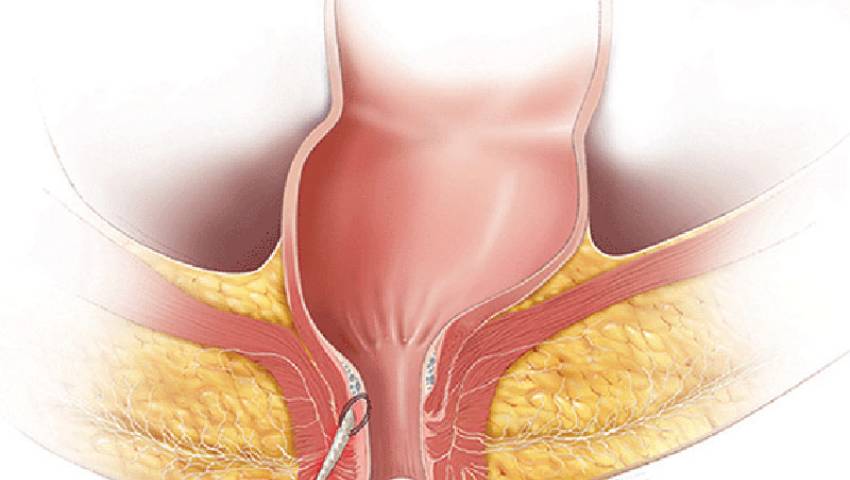
Researchers at the University of Birmingham, in collaboration with clinicians and industry partners, are working on the development of a groundbreaking device that has the potential to revolutionize the clinical management of anal fistula. Anal fistula is a condition in which individuals experience daily discomfort and a foul-smelling discharge from a tunnel that forms between the bowel and the skin surrounding the anus. This condition mainly affects young people and has a significant impact on their employment and family life due to chronic infection and pain.
Diagnosis of anal fistula is quite common, with over 12,000 people being diagnosed in the UK each year. However, the condition is notoriously difficult to treat. The main challenge for clinicians is to allow the fistula tract to drain and heal while preserving continence. Current treatment options involve multiple operations under general anesthesia over an extended period of time, often resulting in hygiene difficulties that can become permanent.
The consortium responsible for the development of the new device consists of experts from University Hospitals Birmingham NHS Trust, the University of Birmingham, and industry partners Neotherix and Keighleycolo. The device, currently known as the Seton-Scaffold Device, offers a less invasive and more effective alternative to existing treatments. It combines a bioresorbable scaffold to facilitate the healing of the fistula with a thin and comfortable seton to achieve drainage.
The material used in the device encourages the migration and settling of the body’s cells into the scaffold, which is the initial step in the healing process. Over several weeks, the scaffold material and seton thread are slowly resorbed by the body. One of the main advantages of this device is that it can be delivered to awake patients, making it a safer and more cost-effective alternative to the current treatment approach.
The consortium’s plans for the future include finalizing the product design, manufacturing sterile devices, and conducting a pivotal clinical study across 10 hospitals in the UK. The study aims to demonstrate the clinical safety and healing outcomes of the device in over 100 patients with anal fistula, including those with Crohn’s disease.
Prior to this project, the consortium conducted education, training, and usability sessions, as well as a study involving 20 patients. The results of this study showed that the device is safe to use and has received positive feedback from both patients and clinicians.
The consortium is now exploring commercialization opportunities and considering the most effective route to market for this innovative and affordable device.
Professor Tom Pinkney, Chief Investigator at the University’s Institute of Applied Health Research, commented, “Our data from a small first-in-man study demonstrated this device could be more effective than anything else on the market and make a real difference to patients’ lives.”
Dr. Mike Raxworthy, CEO of Neotherix, added, “This project will generate critical data to demonstrate the safety and clinical value of the Seton-Scaffold Device and will allow us to move forward with commercial partners. It is an excellent example of the value of strong academic-clinical-industry collaboration in addressing unmet medical needs.”
Professor Mike Keighley of Keighleycolo Ltd, an expert in colorectal disorders, expressed his belief that the device will provide an affordable and effective therapy without morbidity or the need for hospital admission, potentially benefiting patients worldwide.
*Note:
1. Source: Coherent Market Insights, Public sources, Desk research
2. We have leveraged AI tools to mine information and compile it