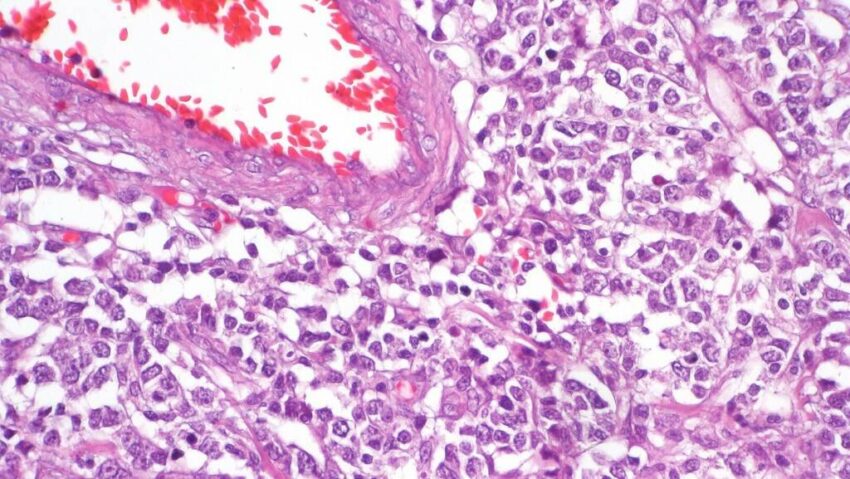
A recent international study led by Stanford Medicine has uncovered the potential of using levels of tumor DNA circulating in the blood to predict treatment response and disease recurrence in Hodgkin lymphoma patients. This breakthrough could allow patients with favorable outcomes to avoid lengthy treatments. Additionally, the study revealed that Hodgkin lymphoma can be classified into two distinct groups, each with different genetic changes and prognoses. These genetic changes could pave the way for the development of new, less toxic therapies that target the weaknesses in the cancer’s growth mechanisms.
Unlike other cancers, Hodgkin lymphoma has been challenging to analyze at the molecular level due to the scarcity of Hodgkin lymphoma cells within the tumor tissue. It is estimated that only about 1% of cells within a large tumor are cancer cells, making it difficult to identify the genetic drivers of the disease. However, the study utilized an optimized DNA sequencing technique called PhasED-Seq, developed at Stanford Medicine, to detect rare fragments of cancer DNA in patients’ bloodstreams. This technique proved to be highly sensitive, capable of detecting as few as one cancer DNA sequence in 1 million non-cancer DNA sequences.
The research team analyzed blood samples from 366 Hodgkin lymphoma patients using PhasED-Seq and another technique called CAPP-Seq. Surprisingly, they found more cancer DNA in the blood samples than in the tumor tissue itself. This breakthrough allowed the researchers to categorize patients into two distinct subgroups based on the genetic changes present in their cancer cells. The first subgroup primarily consisted of younger patients and had a more favorable outcome, with approximately 85–90% of patients surviving for three years without disease recurrence. The second subgroup, which included both younger and older patients, had a less favorable outcome but still had a good prognosis, with approximately 75% of patients living for at least three years without recurrence.
Significantly, both subgroups contained a unique mutation in the gene for the receptor of interleukin 4 and interleukin 13, cellular signaling proteins. This discovery opens up the possibility of exploiting these vulnerabilities in the tumor for therapeutic purposes. Additionally, the researchers found that patients with undetectable levels of circulating tumor DNA shortly after starting treatment were less likely to experience disease recurrence compared to those with residual circulating cancer DNA. This finding confirms the utility of using circulating tumor DNA to predict treatment response and disease recurrence.
The research team hopes that these findings will contribute to improving care for Hodgkin lymphoma patients and eventually lead to a cure for every patient without toxicity. The study involved collaboration with researchers from various institutions, including British Columbia Cancer, University Hospital François Mitterrand, St. Jude Children’s Research Hospital, the Oncology Institute of Southern Switzerland, KU Leuven, the University of Strasbourg, Emory University, the Fred Hutchinson Cancer Research Center, the Hospices Civils de Lyon, and the Université Catholique de Louvain.
Overall, this study represents a significant advancement in understanding the prognosis and biology of Hodgkin lymphoma. By leveraging the power of circulating tumor DNA, researchers have uncovered distinct genetic changes in the cancer cells and identified potential therapeutic targets. Additionally, the ability to predict treatment response and disease recurrence using circulating tumor DNA could have a profound impact on treatment decision-making and patient outcomes. As further research is conducted in this area, it is hoped that these findings will translate into improved care and increased survival rates for Hodgkin lymphoma patients.
*Note:
1. Source: Coherent Market Insights, Public sources, Desk research
2. We have leveraged AI tools to mine information and compile it